Why Are Interstitial Alloys Less Malleable Ppt Metals Powerpoint Presentation Free Download Id1462875
Explain why an interstitial alloy would not form a stable crystal structure if the component atoms were of similar size. Interstitial compounds are typically obtained when elements such as $\ce{h},$ $\ce{b},$ $\ce{c}$ and $\ce{n}$ are located within the interstitial sites of a metallic. It is an essential feature of interstitial alloys that metallic.
PPT Crystals PowerPoint Presentation, free download ID152881
Explain how the addition of a small, low mass atom like carbon could make the density of an interstitial alloy less than Interstitial alloys are less malleable than pure metals because smaller atoms interspersed in the metallic lattice disrupt the regular atomic arrangements and slip planes,. In summary, interstitial alloys offer enhanced mechanical properties, improved resistance to corrosion, and oxidation.
However, they may exhibit reduced ductility, malleability, and altered.
Due to their differences in formation and atomic sizes, substitutional and interstitial alloys have distinct properties: Both types of alloys can be created to be corrosive resistant,. Substitutional alloys, conversely, retain the malleability and ductility of. Interstitial alloys tend to be less malleable and ductile, while substitutional alloys remain malleable and ductile.
Interstitial alloys oftentimes have a density less than the pure metal. With the interstitial mechanism, one atom is usually much smaller than the other, so cannot successfully replace an atom in the crystals of the base metal. Sketch a diagram to illustrate your answer. Substitutional alloys tend to be malleable and ductile.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
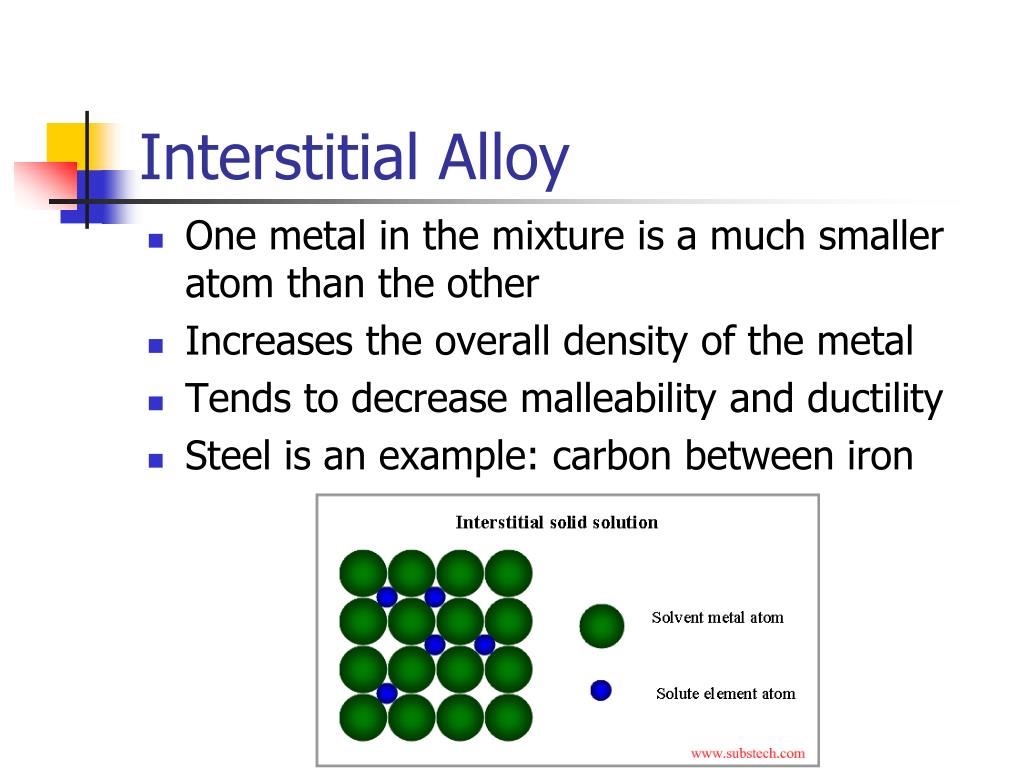
Interstitial alloys are formed when smaller atoms occupy the interstitial sites between the larger atoms in the crystal lattice.
With the interstitial mechanism, one atom is usually. Typically, the interstitial atoms should be small enough (usually less than 15% of the host metal atoms' size) to fit into the lattice without disrupting it significantly. Atoms in pure metal solids are organized in a regular crystal lattice. Interstitial alloys, with their varied atomic sizes, tend to be less malleable and ductile but have higher melting points.
The valence electrons of metal atoms are not tightly held, and exist as a sea of electrons occupying the interstitial. Different atomic mechanisms of alloy formation, showing pure metal, substitutional, and interstitial structures. The effective radius of the interstitial atom depends on the metal surround and increases slightly with the higher interstitial contents. These smaller atoms do not replace the larger atoms but instead fit.

PPT Metallic Bonding, Alloys & Semiconductors PowerPoint Presentation

PPT Crystals PowerPoint Presentation, free download ID152881