Caco A Covalent Of Ionic Bond Covlent Chrcter W3schools
This is because calcium is a metal and carbonate is a polyatomic ion, so they form an ionic bond due to the. The compound itself is ionic, but in reality, it. To determine which bond is stronger, start by identifying and listing the electronegativities of calcium (ca), carbon (c), and oxygen (o).
2 Ionic and covalent bonds YouTube
Calcium carbonate is made of carbonate and calcium. The bond between calcium (ca) and carbonate (co3) is ionic. Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds.
In this article, we will delve into the intricate world of chemical structures to determine whether caco3 is ionic or covalent.
The simplist guide to the covalent or ionic. Calcium carbonate (caco 3) is a compound with both ionic and covalent bonds. Carbonate is a compound of carbon. P i = 0 indicates a pure covalent bond while p i = 1 indicates a purely ionic bond.
Here, calcium carbonate has an ionic bond between the calcium cation (ca 2+) and the carbonate anion(co. The ionic bond between the calcium ion and the carbonate ion ensures the stability of the compound, while the polar covalent bonds within the carbonate ion contribute to. Calcium carbonate is an ionic compound featuring calcium ions (ca²⁺) and carbonate ions (co₃²⁻), connected by strong ionic bonds. Specifically, covalent bonds can be formed by combining.
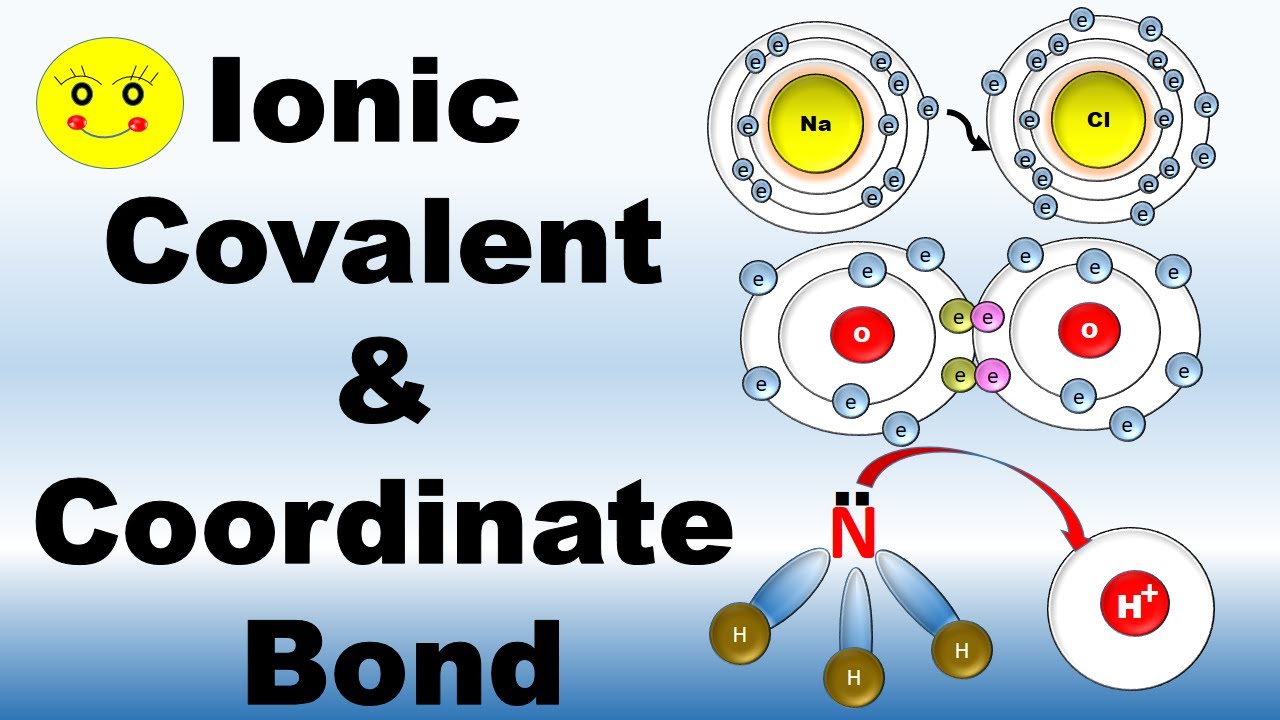
Ionic bond ,Covalent bond and Coordinate bond Chemistry Types of
The presence of ionic bonds in compounds like \text{caco}_3 can be seen in electrostatic attraction between charged ions, while polar covalent bonds can be observed by.
Think about it in parts. Naming covalent compounds in the previous section, the process for using a lewis structure to determine the chemical formula of a covalent molecule was presented and. The bond between calcium (ca) and carbonate (co₃) ions is primarily ionic. The result of this type of interaction is called a covalent, or molecular, bond.
Covalent bonding is where two or more atoms share valence electrons to complete their orbital. The degree to which electrons are shared between atoms varies from completely equal (pure covalent bonding) to not at all (ionic bonding). Calcium carbonate has both ionic and covalent bonds. There are multiple types of chemical bonding, both inter molecular and intra molecular, that can exists between atoms.

chemical bonding Ionic and covalent compounds Britannica
In the compound caco₃ (calcium carbonate), the types of bonding present are:
Covalent bonds are the strongest bonds between atoms found in chemistry. The ionic bond is formed between the ca2+ cation and the. This type of bonding is. Calcium, being a metal, tends to lose two electrons to achieve a stable electron configuration.
As a chemistry expert, it is important to provide. C1 ionic and covalent bonding chemistry guided notes learning block objectives in this learning block, you will explore how electrons are transferred in the formation of ionic bonds. The bond between calcium (ca) and carbonate ions is ionic.
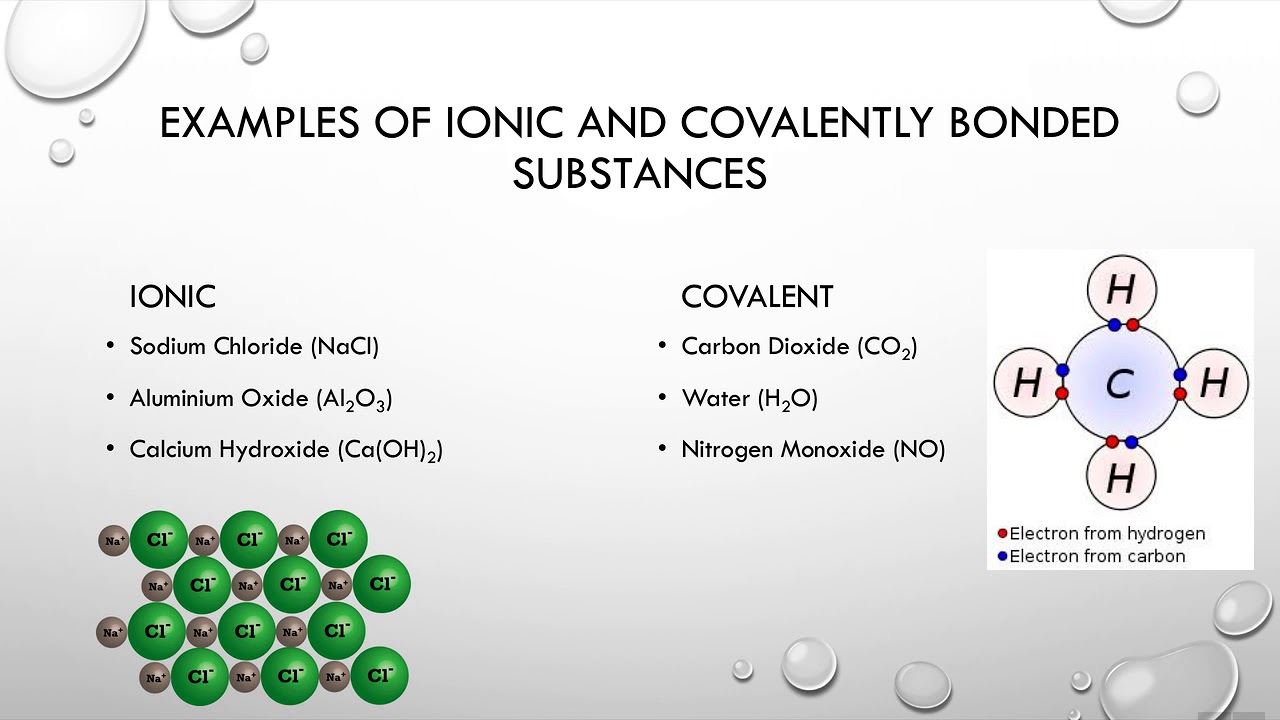
2 Ionic and covalent bonds YouTube