Where Is Majority Of Negative Charge On The Water Molecule Ppt Properties Powerpoint Presentati Free Download Id
This occurs due to the unequal sharing of electrons, making oxygen partially negative while hydrogen is. A water molecule consists of two hydrogen atoms and one oxygen atom. Study with quizlet and memorize flashcards containing terms like how many hydrogen atoms are in a molecule of water?, how many oxygen atoms are in a molecule of water?, what holds the.
Complete Water Molecule Diagram With Partial Changes And Dip
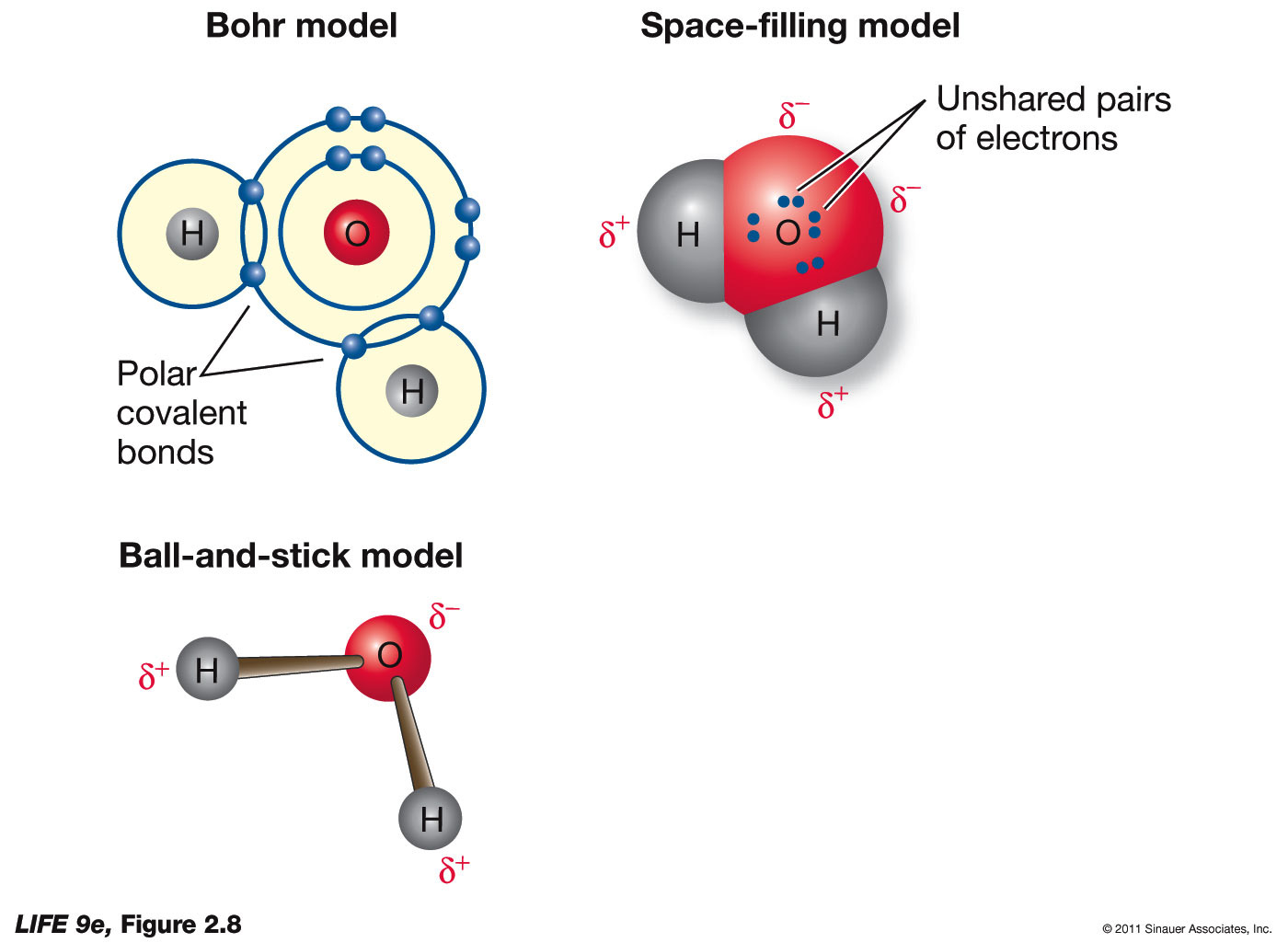
Most of the negative charge is located in the oxygen atom of the h₂o water molecule. Where is the majority of negative charge on the water molecule? The oxygen atom in a water molecule attracts negatively charged electrons more strongly than the hydrogen atoms.
The larger gray shaded circles represent.
The majority of the negative charge on a water molecule is found on the oxygen atom. The small, unshaded circles represent. One of the strands has a negative charge and another strand has a positive charge. Where is the majority of negative charge on the water molecule?
The majority of the negative charge in a water molecule is around the oxygen atom. One of the strands has a negative charge and another strand has a positive charge. This tiny force of attractions is called a. In the h₂o water atom, the component oxygen is more electronegative than hydrogen.
The Charge On 500cc Of Water Due To Protons
Oxygen is more electronegative than hydrogen, so the shared electrons in the covalent bonds are.
Hydrogen and oxygen would like to have stable electron configurations but do not as individual atoms. Because the bonds carry a small amount of delocalized charge, which is transferred when the bonds are broken or formed, changes in ph could affect charge transfer. To balance the negative charge of 8 (2+6) electrons, the oxygen nucleus has 8 protons. Slight positive charges on the hydrogen atoms in a water molecule attract the slight negative charges on the oxygen atoms of other water molecules.
The hydrogen side of the water molecule has a slight positive charge. The positive area charge (hydrogen) of one water molecule is attracted to the negative area (oxygen) of a different water molecule. Some molecules that are covalently bonded do not have a difference in charge across the molecule. Hydrogen positive charges in new molecules are attracted to oxygens negative molecule.

Complete Water Molecule Diagram With Partial Changes And Dip
The majority of negative charge on the water molecule is present around the oxygen atom.
This weak attraction is often Two hydrogen atoms and one oxygen atom are present in water. Oxygen this causes a region of slight negativity (although. On the other side of the molecule, a negative charge exists.
There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. A greater negative charge is around the oxygen atom than around the hydrogen atoms (because the shared electrons are. What kind of charge would oxygen atom. The majority of the negative charge on the water molecule is located on the oxygen atom.

Complete Water Molecule Diagram With Partial Changes And Dip
However, oxygen is more electronegative than most other elements, so the oxygen atom has.
Which part of a water molecule is delta negative? This gives water an asymmetrical distribution of charge.