Does Ch4 Form Hydrogen Bonds Solved Both Structures Below Are Symbolic Representations For The
Hydrogen bonding occurs where hydrogen is bound to a strongly electronegative. For example, does ch4 form hydrogen. But like you mentioned since fluorine is more en than nitrogen, hf should have stronger imfs and thus a higher boiling.
Can Ch4 Form Hydrogen Bonds CANZI
Methane, being a hydrocarbon molecule, does not have a hydrogen atom bonded to a highly electronegative. No, ch4 (methane) does not exhibit hydrogen bonding because it does not contain hydrogen atoms bonded directly to highly electronegative elements like oxygen,. The tetrahedral arrangement of the bonds in ch4.
It can be used as a gaseous fuel because it yields vast amounts of energy if it is.
Ch4, or methane, is the smallest and most simple of all alkane molecules. The short answer is no, ch4 cannot form hydrogen bonds. Why are hydrogen bonds associated with water (h2o), but not with methane (ch4)? Does ch4 have intermolecular hydrogen bonding?
The molecule that will not form hydrogen bonds is ch4 (methane) because it does not have hydrogen atoms bonded to highly electronegative atoms like oxygen, nitrogen, or. Hf and nh3 can both form 2 hydrogen bonds. Ch4 cannot form hydrogen bonds. The question is asking whether methane (ch4) is capable of forming hydrogen bonds.

Hydrogen bonds What are they? ppt download
This requires an understanding of hydrogen bonding and the characteristics of the ch4 molecule.
It does not have any. This is because hydrogen bonds are a type of electrostatic interaction, which is only possible in molecules in. No, ch4 (methane) does not exhibit hydrogen bonding because it does not contain hydrogen atoms bonded directly to highly electronegative elements like oxygen, nitrogen, or. For example, does ch4 form hydrogen.
Use the electronegativity table shown here to determine which of the following molecules is likely to form hydrogen bonds with other identical molecules. To answer this, we need to consider the molecular structure of ch4 and the nature of hydrogen bonds,. Can ch4 form hydrogen bonds? Use the electronegativity table shown here to determine which of the following molecules is likely to form hydrogen bonds with other identical molecules.

Can Ch4 Form Hydrogen Bonds CANZI
The question is asking whether methane (ch4) can form hydrogen bonds.
Given the molecules ch4, nah, nh3, bh3, and hi, only nh3 (ammonia) can form hydrogen bonds.
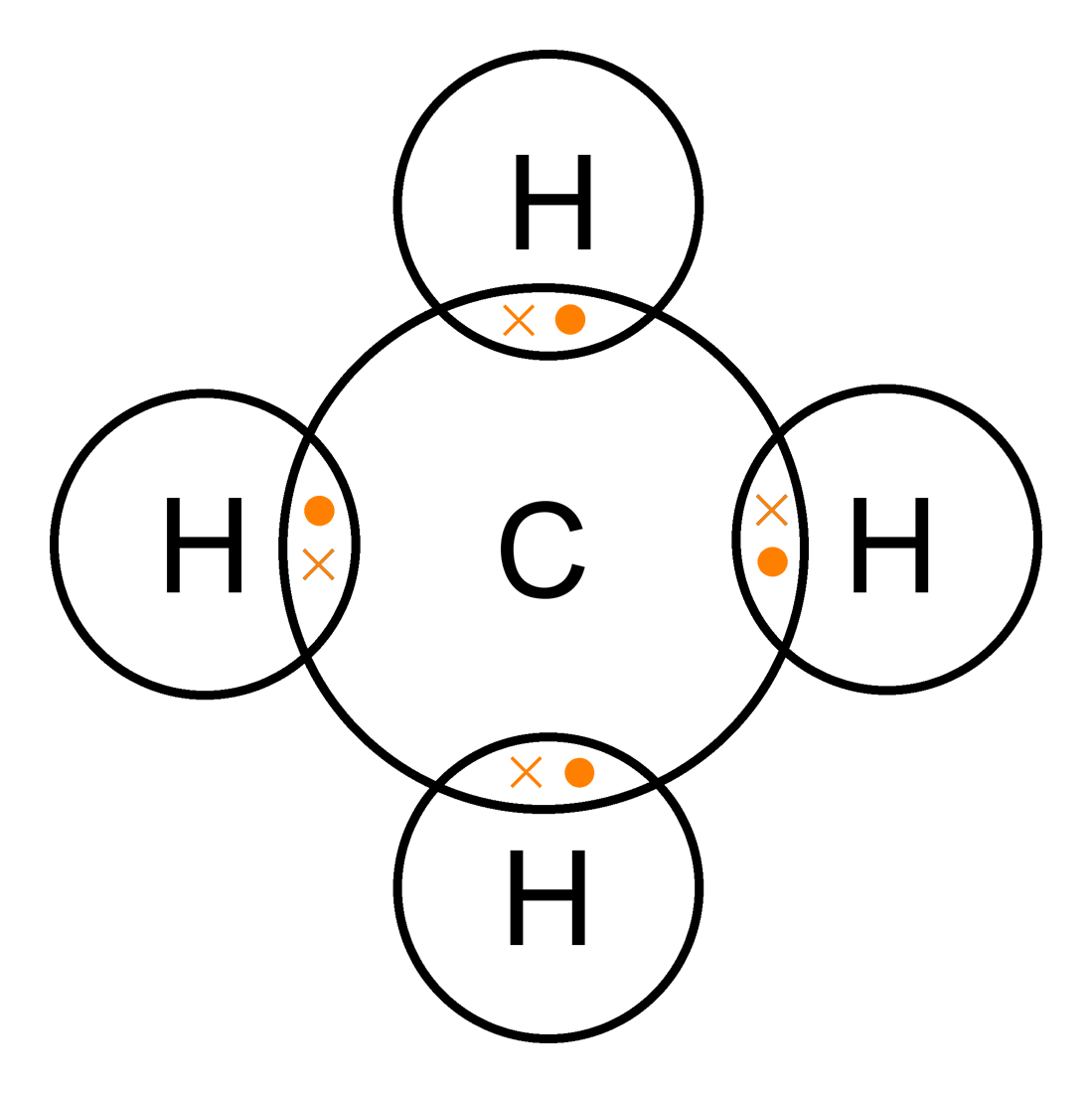
What Is The Correct Lewis Dot Diagram For Ch4