Lewis Structure For N Ch3 3 Koc Youtube
Lewis structures are representations that show the bonding patterns of a compound. Contact pro premium expert support » compute answers using wolfram's. In n (c h 3) 3, lone pair on n remains on it causing l p − b p repulsion while in n (s i h 3) 3, lone pair is transferred.
N(ch3)3 Lewis Structure
What is the lewis structure of [//substance:n (ch3)3//]? N (c h 3) 3 is pyramidal but n (s i h 3) 3 is planar. A lewis structure is a way to show how atoms share electrons when they form a molecule.
For the lewis structure of individual elements,.
Lewis structures show all of the valence electrons. In the lewis structure of n (ch3)3 structure there are a total of 26 valence electrons. (1)writing valid lewis structures for the constitutional structure of molecular substances for a given composition. N (ch3)3 is also called trimethylamine.
The above lewis structure displays a total of 7 valence electrons. Enter the formula of the molecule in the field provided for it. This widget gets the lewis structure of chemical compounds. Enter a chemical element or formula to calculate and draw its lewis dot structure.

How to Draw the Lewis Dot Structure for N(CH3)3 Trimethylamine YouTube
To use the lewis structure calculator follow these steps:
These show the valence electrons involved in bonding and those that are. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. To find the lewis structure for n (c h 3) 3, calculate the total number of valence electrons by summing the valence electrons of nitrogen, carbon, and hydrogen atoms. The carbon atom (c) is at the center and it is surrounded by 3.
This results in a trigonal. For $\mathrm{n}\left(\mathrm{ch}_{3}\right)_{3}$, nitrogen has 5 valence electrons, each carbon has 4, and each hydrogen has 1, giving a total of 26. There are 5 valence electrons on nitrogen; N (c h 3) 3 and n (s i h 3) 3 both have s p 3 − hybridized n − atom and pyramidal structure.
N(ch3)3 Lewis Structure
The most preferred lewis structure of ch 3 is as shown below.
Have a question about using wolfram|alpha? For example, if we want to obtain the lewis structure of the sulfate ion,. Be sure to use the proper capitalization for all element symbols.
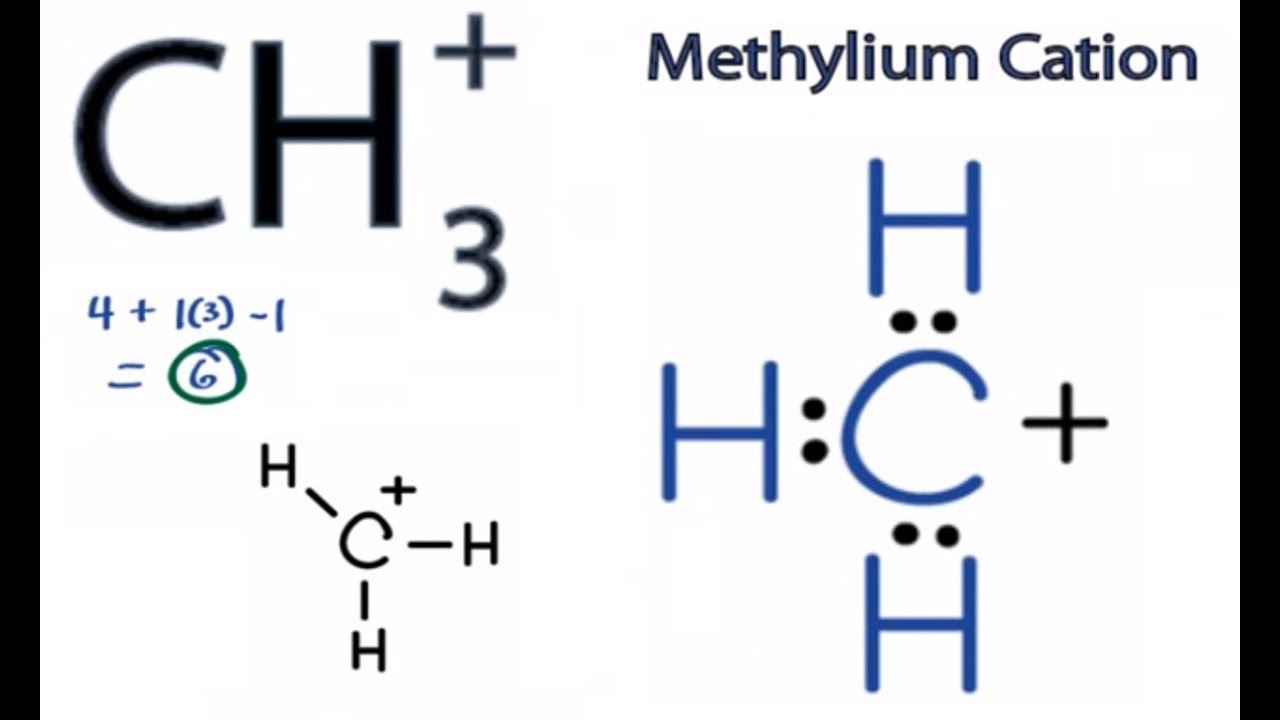
CH3+ Lewis Structure How to Draw the Lewis Structure for CH3+ YouTube